ABOUT US
LIGHTHOUSE offers complete solutions for headspace Oxygen Monitoring, Container Closure Integrity Testing, Moisture Determination and Microbial Contamination inspection of media vials. Our solutions include feasibility studies, process and packaging studies, method development, method validation protocols, automated or benchtop lease platforms, on-site trouble shooting and support.
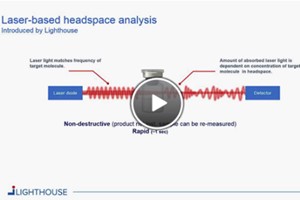
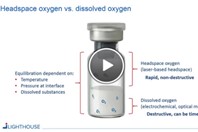
Rapid, nondestructive, laser-based headspace analysis is suitable for applications specific to the sterile pharmaceutical industry. It is a useful analytical tool to generate statistical process and product data in all stages of the product life cycle, from Development to Manufacturing and Quality Control. LIGHTHOUSE Instruments is the leading provider and manufacturer of headspace analysis platforms and measurement services.
Contact us for a free demo, feasibility study, or other information.
CONTACT INFORMATION
Lighthouse Instruments
2030 Avon Court
Charlottesville, VA 22902
UNITED STATES
Phone: 434-293-3081
Contact: Sales
FEATURED APPLICATION NOTES
-
This application note describes how laser-based headspace analysis is used for the rapid non-destructive determination of headspace oxygen levels in pre-filled syringes. Data is presented demonstrating two major applications of this technique: 1) headspace oxygen monitoring on a pre-filled syringe line filling oxygen-sensitive product, and 2) container closure testing of pre-filled syringes.
-
Learn how rapid, nondestructive laser-based headspace analysis can support data generation to demonstrate the maintenance of seal integrity during deep cold storage and transport.
-
This app note describes the application of the PULSAR inspection platform to perform 100% headspace oxygen monitoring during the filling of oxygen-sensitive formulations, 100% container closure inspection of suspect batches, moisture inspection of freeze dried product, and automated media fill inspection.
FEATURED CASE STUDIES
-
A new biological product, in pre-filled glass syringes, had demonstrated oxygen sensitivity in stability studies. Therefore, the headspace was purged with nitrogen during filling, and the client wanted to validate the batch production process and assess the nitrogen purge efficiency. In this study we demonstrate by performing a 100% inspection on the engineering batches using non-destructive headspace oxygen analysis you could gather the data needed before a commercial launch.
-
Rapid non-destructive headspace moisture analysis from LIGHTHOUSE enables fast moisture determination of statistical numbers of lyo samples.
-
During QC testing, a number of vials of liquid vaccine product stored at -80°C were found to have an overpressure, representing a serious safety risk and they needed to identify the root cause.
WHITE PAPERS
-
Laser-based headspace analysis is a non-destructive and rapid method for testing container closure integrity. We demonstrate that headspace analysis is equally as sensitive as helium leak rate testing.
-
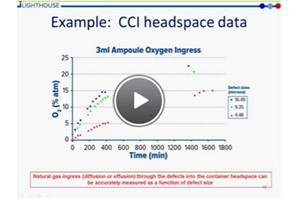
To ensure patient safety, good container closure integrity (CCI) is of great importance for all sterile injectable products. Recent regulatory guidance has made clear that there is no ‘gold standard’ for CCI testing. As a CCI test method, headspace analysis is based on detecting changes in the headspace gas composition that result from gas ingress through a leak. Non-destructive headspace analysis, using laser-based spectroscopy, can be used to directly quantify the gas concentration inside a sealed parenteral package. It can be applied to a range of product configurations, and formulations, and has historically been used for detecting leak defects in modified headspace product.
-
Recent regulatory guidance has triggered changes in industry best practices in the area of container closure integrity testing (CCIT). However, assuring good CCI of sterile injectable product goes beyond CCI testing. A more science-based holistic approach that includes robust design & qualification of the process and the implementation of appropriate process controls is required. This article describes a framework enabling such a holistic approach to CCI that assures both the primary packaging and the process contribute to good CCI of sterile injectable vial product.
-
Non-sterile pharmaceutical products, including oral solid dosages (OSDs) such as tablets, capsules, and softgels, are subject to microbiological examination prior to release and during stability testing.
-
This paper presents three case studies to show that direct water activity measurements can determine the impact of moisture on potency and dissolution.
-
Controlling water content in oral solid dosage (OSD) products, and dry pharmaceutical products in general, is essential to maintaining efficacy and safety. Measuring the water activity at multiple time points during the product life-cycle will correlate to changes in critical quality attributes such as degradation of the active ingredient, changes in the dissolution or disintegration rate, and changes in physical properties such as hardness or friability.
-
This article summarizes the current state of container closure integrity testing in the pharmaceutical and biopharmaceutical industries and outlines possible approaches for developing a CCIT strategy.
-
Rapid water vapor determination with an optical method could replace the slow destructive traditional methods for the moisture analysis of freeze-dried product. A description of industry applications of headspace moisture analysis including freeze drying cycle optimization, lyo chamber moisture distribution mapping, and 100% moisture inspection of commercial freeze-dried product.
-
Gain insight into the process, ensure the maintenance of sterility for finished product after capping, and meet current regulatory guidance using laser-based headspace inspection.
CASE STUDIES
-
Explore how a non-destructive method can be developed to test container closure integrity in autoinjectors equipped with an optically transparent window.
-
Here, we investigate possible primary packaging solutions for therapies requiring even colder storage temperatures down to cryogenic levels (-150°C to -180°C).
-
A new biological product, in pre-filled glass syringes, had demonstrated oxygen sensitivity in stability studies. Therefore, the headspace was purged with nitrogen during filling, and the client wanted to validate the batch production process and assess the nitrogen purge efficiency. In this study we demonstrate by performing a 100% inspection on the engineering batches using non-destructive headspace oxygen analysis you could gather the data needed before a commercial launch.
-
The accurate determination of oxygen concentration as a critical quality parameter for oxygen-sensitive products is important across the product life cycle activities. Traditional methods for determining headspace oxygen levels in parenteral containers, such as electrochemical methods or gas chromatography, are slow and destructive. This article describes several case studies comparing non-destructive laser-based oxygen headspace analysis with electrochemical oxygen analysis and gas chromatography.
-
This case study describes how packaging development and process study data of a pharmaceutical vial product requiring deep cold storage can be combined in a holistic approach.
-
With the application of non-destructive headspace moisture analysis, small-scale proof of concept studies can minimize project risk and the number of full-scale runs in the freeze-drying validation process.
-
A gene therapy clinical trial was halted due to CCI issues in deep cold storage. A CCI test method was developed that enabled non-destructive CCI testing of product vials at these cold temperatures.
-
A manufacturer of a syringe product received complaints about discolored product that was nearing the end of shelf life. A root cause investigation was started and product syringes were put on stability. The headspace oxygen levels were monitored over time. The laser-based headspace analysis proved to be a useful tool to check package integrity and for the presence of reactive headspace gases that can degrade the formulation.
-
A client had an existing filling line and wanted to optimize the nitrogen purge process to decrease headspace oxygen levels to 2%. In addition, frequent line stoppages resulted in a need to identify and reject high oxygen vials that had lost the nitrogen headspace during the stoppage. A purging process qualification study was performed using rapid non-destructive headspace oxygen analysis in an at-line set-up with samples being measured immediately from the line.
-
Regulators are paying closer attention to the proper design of robust Container Closure Integrity (CCI) studies and the validation of CCI test methods.
-
Vacuum in finished sterile product containers is critical for certain pharmaceutical formulations to ensure proper reconstitution before administration to the patient, or to prevent interactions between the formulation and headspace gas. A loss of vacuum can be a clear indicator of a container closure integrity defect or an issue with the original sealing process. Using a LIGHTHOUSE headspace analysis platform for non-destructive headspace pressure determination verifies the maintenance of vacuum while preserving the product sample.
-
During QC testing, a number of vials of liquid vaccine product stored at -80°C were found to have an overpressure, representing a serious safety risk and they needed to identify the root cause.
-
Rapid non-destructive headspace moisture analysis from LIGHTHOUSE enables fast moisture determination of statistical numbers of lyo samples.
-
In cases where oxidation of the formulation causes discoloration and eventual degradation of the product, non-destructive headspace analysis tests can give deep insight into the root cause. Since the samples are not destroyed by the headspace analysis, further testing can be done to accurately correlate headspace conditions with other product characteristics.
-
A leading contract manufacturer approached LIGHTHOUSE for help after a suspected raised stopper issue motivated the manufacturer to place several batches into quarantine. A decision was made to perform 100% container closure inspection of the product vials with the help of LIGHTHOUSE.
FEATURED WEBINARS
-
Increased regulatory scrutiny and exciting new analytical technologies have altered the landscape of container closure integrity testing. In order to provide guidance to this new environment the US Pharmacopoeia revised Chapter <1207> Sterile Product Package Integrity. View this webinar recording to learn about the new guidelines and how they will impact your approach to sterile product package integrity.
-
This webinar presents how to design and conduct studies to assess the total oxygen permeation rate of your pre-filled syringes, and how to determine if the permeation is primarily through the plunger or through the tip. Protecting oxygen-sensitive formulations during filling will also be discussed.
-
This webinar will review how oxygen levels in finished parenteral drug containers can be determined and controlled throughout the product life cycle by using laser-based headspace analysis.
-
This webinar describes how non-destructive headspace moisture analysis can be used for characterizing batch moisture distributions, for lyo cycle development and optimization, and for freeze dryer moisture mapping and validation.
-
Dr. Derek Duncan, Director of Product Lines, Lighthouse Instruments, covers approaches that can be used for method development for CCI testing in all phases of the product life cycle.
-
The new language in EU Annex 1 will likely have a significant impact on your CCI testing practices. In this webinar CCI testing strategies and the proposed revisions to EU Annex 1 are discussed.
PRODUCT NOTES
- FMS-Moisture/Pressure Headspace Analyzer
- FMS-Carbon Dioxide Headspace Analyzer Datasheet
- Integra Laboratory Vial Crimper
- Residual Seal Force Tester
- Container Closure Integrity Testing Using Gas Ingress
- Pharmaceutical Water Activity Analyzer: FMS Brochure
- Container Closure Integrity Testing Services
- FMS-Water Activity Headspace Analyzer Datasheet
- Determine Feasibility Of Headspace Analysis On Your Product Samples
- FMS-Oxygen Headspace Analyzer Datasheet
- PULSAR 100% Headspace Inspection System
- Headspace Measurement Services
- Annex 1-Compliant Cold Storage Package System Qualification
- Compliance Program For EU Annex 1 Container Closure Requirements