Static And Dynamic Light Scattering for Determining Absolute Molecular Weight And Hydrodynamic Radius Of Biomolecules
John P. Helfrich, Precision Detectors Inc.
Contents
Introduction
Light Scattering Technology
Biomolecule Applications
Bovine Serum Albumin (BSA)
Therapeutic Protein (Confidential Structure)
Monoclonal Antibody
DNA-Plasmid
Conclusions
References
Introduction (Back to Top)
Molecular weight and/or molecular weight distributions are fundamental parameters for characterizing macromolecular biomolecules such as proteins, polysaccharides, oligonucleotides, and antibodies. Traditional analytical methods for molecular weight determinations include size exclusion chromatography (SEC), gel electrophoresis, and, more recently, Rayleigh light scattering and time of flight mass spectrometry (MALDI-TOF-MS). Despite the fundamental nature of average molecular weight, determining its value is often hampered by sample heterogeneity, polydispersity, thermodynamic non-ideality, and, in many cases, self-association. Very often, large biomolecules will aggregate as a function of temperature, pH, ionic strength, and concentration. These effects cause conformational changes in the molecular structure that can alter the biomolecules' function as therapeutic or diagnostic agents. Understanding these insights is fundamental in the research, development, and process production/quality control of today's biomolecules.
Rayleigh or static laser light scattering detectors have been used for over a decade to determine the molecular weight characteristics of industrial polymers such as polystyrene, polycarbonate, and polyolefins (polyethylene and polypropylene). Recent innovations in modern high-speed electronic components such as high performance diode lasers, high-speed digital signal processors, and modern avalanche photodiode detectors has lead to the evolution of a new dynamic laser light scattering detector. This combination static and dynamic light scattering detector has a 10 µL flow cell design and is capable of characterizing both molecular weight and size for biomolecules eluting from modern HPLC/SEC instruments. This new detector and associated software provides:
- Absolute molecular weight data for each eluting component.
- Hydrodynamic Radius (Rh)—from DLS data.
- Rh with molecular weight distribution data provides insights on molecular shape and conformation.
- High sensitivity aggregation and self-association determination.
Light Scattering Technology (Back to Top)
When a polarized, mono-chromatic laser beam passes through a solvent containing biomolecules, the excess light scattered by the molecules at an angle to the incident beam over that scattered by the solvent alone is directly proportional to the molecular weight (Mw) times the concentration of the biomolecule. For most protein-based biomolecules, a single collection angle of 90º is all that is necessary for determining accurate molecular weights (1). For very large molecules (generally greater then 800 kD) collecting the scattered laser light at two angles (90º and a low angle of 15º) will provide an accurate molecular weight and a calculation of the radius of gyration (Rg). In addition, as biomolecules pass through the 10 µL flow cell, they undergo Brownian movement that is related to their hydrodynamic radius (Rh) according to the Stokes-Einstein equation. Through the use of a high-speed photon counting detector mounted at a 90º angle from the incident laser beam, the diffusion constant is calculated from the decay time of the autocorrelation function of the scattered light. This is similar to the doppler-shift effect of sound frequencies emitting from a moving source. From the diffusion constant, Rh is then calculated. The key characteristic of this new DLS technology is its very high sensitivity and unique ability to operate in a flow-mode with the HPLC/SEC system and calculating Rh at each elution slice during the chromatographic separation.
Using this "absolute" detector as an orthogonal technique to traditional SEC data provides confirmation of data for regulatory submissions and compliance purposes. An outline of this novel combined dynamic and static laser light scattering platform is seen in Figure 1.
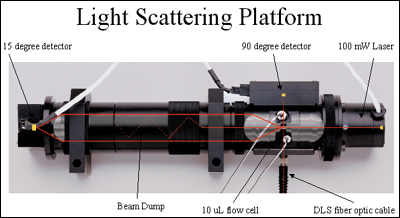
Biomolecule Applications (Back to Top)
Dynamic light scattering detection can be applied to most soluble biomolecules such as proteins, large polypeptides, polysaccharides, oligonucleotides, DNA-plasmids, and antibodies having a molecular weight generally greater than 10kD. The detector is fully capable of characterizing molecules having an Rh in the 1.5 nm to 1000 nm range. If molecular weight distributions are the only information needed, the static light scattering detector can "see" down below 5kD.
Many applications involve characterizing protein aggregation and/or conformation variations as a function of time, temperature, pH, glycosylation, etc. In addition, critical regulatory questions concerning the "integrity" of the eluting protein from a SEC column can be clearly elucidated by determining the hydrodynamic radius of the eluting fractions across the entire peak. Any perturbation in the Rh across the peak, as it elutes, reflects a co-eluting contaminant or conformational structure variation of the molecule. The following applications will outline typical data obtained with "flow mode" dynamic light scattering detection.
Bovine Serum Albumin (BSA) (Back to Top)
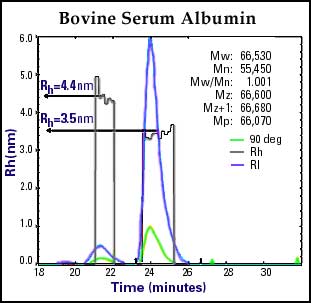
In Figure 2, Bovine Serum Albumin, is characterized in less then 30 minutes by utilizing a refractive index detector and simultaneous static and dynamic laser light scattering (DLS) detectors. The high sensitivity of the DLS detector (with only 160 µg injected) clearly revealed the presence of a well-defined aggregation component in the range of dimer, and a minute amount of trimer. In addition, the "on the fly" autocorrelation function calculated the Rh of the monomer and dimer to be 3.5 nm and 4.4 nm respectively at the same time as the absolute molecular weight is determined. The dimer was determined to be approximately 10% of the total mass, thus representing only 16 µg of material being "seen" and analyzed by DLS.
Therapeutic Protein (Confidential Structure) (Back to Top)
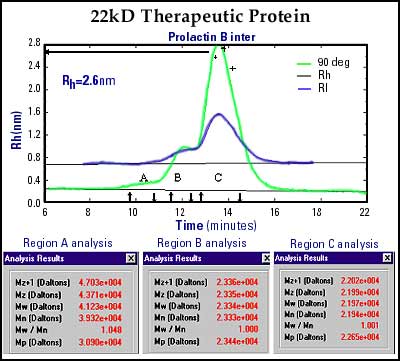
Figure 3 is an SEC separation of a proprietary 22kD therapeutic protein. This QC chromatogram and DLS analysis reveals three components. Region C is the major component with a molecular weight determination (via static light scattering at 90º) of 22kD. In addition, the "on-the-fly" size calculation indicates a Rh of 2.6 nm. This correlation of Mw and Rh is useful for enhanced quality control data. Region B was initially thought to be the dimer of the main component, however, its molecular weight of only 23kD (by light scattering) indicated that it is most likely a glycosylated adduct of the main protein. Region A, however, does indicate a dimer as the molecular weight was determined to be 42kD, as expected for the dimer structure.
Monoclonal Antibody (Back to Top)
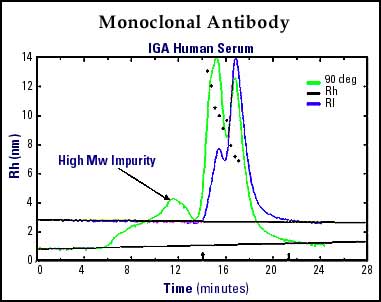
Monoclonal Antibodies are one of the most studied biomolecules due to their complexity and subtle changes in bio-activity as a function of conformational variations. Simple conformational changes due to glycosylation variations can cause significant pharmacological and immunoreactive effects. The use of DLS to characterize monoclonal antibodies allows the "real-time" calculation of hydrodynamic radius across the entire moleclular weight distribution of the monoclonal antibody. Figure 4 shows a human monoclonal antibody separated by SEC with direct elution into a dynamic light scattering detector. The DLS has a higher sensitivity to the larger molecular weight component with the associated Rh values (in nm) plotted across the entire biomolecule. Insights into changes of Rh as a function of various conditions (pH, temperature, concentration, etc.) allow researchers to better understand pharmacological activity, and allows quality control departments to insure the consistency of their product/process for regulatory submissions.
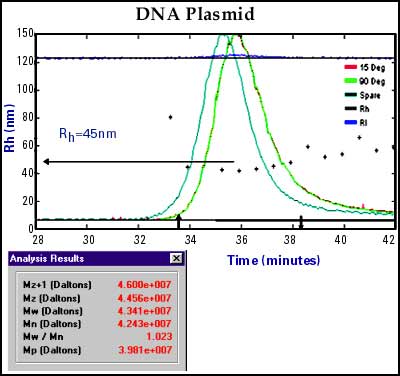
DNA-Plasmid (Back to Top)
Major research in the gene therapy field is focused on the construction and delivery of protein-producing materials directly into target cells with the hope of combating diseases. These DNA-plasmids are ultra-large molecules. Figure 5 is an SEC/DLS chromatogram of a DNA-plasmid showing a uniform Rh of approximately 45 nm across the entire separation. The static light scattering data indicated an extrapolated molecular weight of over 43 million.
Conclusions (Back to Top)
Dynamic laser light scattering detection can easily be added to any HPLC/SEC/FPLC instrument currently performing molecular weight determinations of biomolecules. This high sensitivity detection coupled with "on the fly" determination of hydrodynamic radius (Rh) allows new insights into the conformation and consistency of modern biomolecules.
The unique design of combining both static and dynamic light scattering into one cell provides a new tool to obtain critical path information for biomolecules. These insights will help research departments to develop improved pharmacologically active compounds, and quality control departments to better control the consistency of the commercialized product. DLS is a new research and quality control tool for 21st century biomolecular characterization.
- Practical on-line determination of biopolymer molecular weights by high performance liquid chromatography with classical light scattering detection, Dollinger et.al, Journal of Chromatography, 392 (1992), 215-228.
For more information: John P. Helfrich, Director, Life Science Group, Precision Detectors Inc., 10 Forge Park, Franklin MA 02038. Tel: 508-520-8765. Fax: 508-520-8772. Email: john@lightscatter.com.
